It's time to pick up where I left off last month with the yeast glucose versus oleate network. In that first post, I introduced the network, and then I showed how the target node rows can be logically arranged in an order so that targets with the same combination of inputs are grouped together.
But I wrapped up that introductory post after showing the two different experimental conditions as two separate networks. But by using the link grouping feature, we can create a single combined network that allows us to directly compare the two conditions side-by-side, and that's the topic of this posting.
First, you should go and review how to set up link groups; I covered that in my Caltech dorm post, and so I won't cover that ground again in detail. In this case, I simply created a single .sif file by combining the results from the two different conditions. For the glucose condition links, I added a "-g" suffix to the link tag, while I added an "-o" suffix to the oleate condition links. I then used these two tags to create the link groups. By putting the glucose tag first in the list of groups, I ensured that the edge wedges for the glucose condition would always show up to the left of the oleate condition edge wedge.
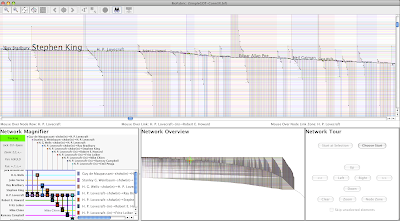
I also created a node attribute file that I used to order the nodes, just like I did in my two original networks. Since there are four transcription factors, there are (4 x 4) - 1 = 15 possible non-zero combinations of the inputs. If you recollect from my first post, I showed how we could consider these 15 different input combinations simply as binary numbers, and then order the targets by just sorting those numbers. This put the target nodes with all four inputs in the topmost rows, and the nodes with only an Adr1p (A) input in the lowest rows. I did exactly the same thing in this case, though in this case I have (4 x 4) x (4 x 4) - 1 = 255 possible non-zero combinations across the four inputs for the two different experimental conditions. Here's the result:
 |
| Click on picture to enlarge |
Let's get oriented here. The leftmost edge wedge contains the links for the targets of Oaf1p (O) under the glucose condition; the next wedge to its immediate right contains links for Oaf1p targets under the oleate condition. Most, but not all, of the O-glucose targets are O-oleate targets too, and there are a whole bunch more new O-oleate targets as well. The pattern of glucose wedge followed by the oleate wedge for each of the four transcription factors is the direct result of our using link grouping to organize the two different experimental conditions. Thus, the same pattern of glucose followed by oleate links repeats across the remaining three source nodes Oaf3p (Y), Pip2p (P), and Adr1p (A).
The crucial point here is to note how we can now directly compare the networks for the two separate conditions. For example, as I just alluded to above, looking at the O targets for glucose versus oleate, we see that maybe 20% of the O targets under glucose are not O targets in the oleate condition. For any combination of inputs and conditions, we can quickly scan the network to find such patterns.
But is the arrangement of node rows that I chose above really the best one for doing these comparisons? I don't think so, and we have complete freedom to arrange the node rows in whatever way works best. The node row ordering I used above simply matched the one I introduced in my last posting. You can see that pattern, starting with the leftmost edge wedge (O-glucose), which has two bands of rows: the band of nodes with edges from O, sitting above the band of nodes without edges from O. That second band of no-edge nodes might require a little bit of imagination to spot, since it's just the empty space below the wedge, but there it is if you think about it a bit! For shorthand, I'll call this banding arrangement (1,0). Then, the next wedge to the right (O-oleate) has four bands (1,0,1,0), the third (Y-glucose) has eight bands (1,0,1,0,1,0,1,0), and and so forth. With this scheme, the rightmost (A-oleate) wedge is the most fragmented, with 256 possible bands (1,0,1,...,0) though there are fewer than that because not all possible combinations are present. Another way to view this arrangement is like a car odometer: the rightmost column is always changing, while the leftmost column almost never changes.
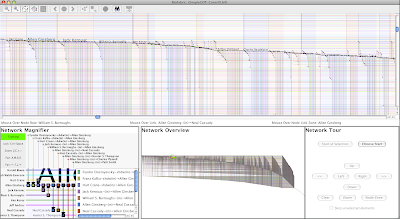
So let's try different node row orders. First, compare the following arrangement with the first. Here, we make the four glucose wedges the most coherent, with the fewest bands, and the oleate wedges are more fragmented:
 |
| Click on picture to enlarge | |
Compare this to the glucose-only network I presented in my introductory post, which I have reproduced here:
 |
| Click on picture to enlarge |
See how the original pattern of the edge wedges is retained? The glucose-only version reappears with this arrangement, it's just interspersed with the oleate edge wedges.
So I like to view the above arrangement as being "glucose condition centric". You can think of it as perhaps the best organization to use if you want to view and think about the changes between the two conditions where the first, glucose condition, serves as the starting point, or baseline.
But perhaps you want to view the two conditions the other way around, where the oleate condition edge wedges are the most coherent:
 |
| Click on picture to enlarge |
Comparing that version to the oleate-only network, that I am again showing here, you can see how the original oleate edge wedges are the ones to retain their shapes in the combined version shown above:
 |
| Click on image to enlarge |
So again, this version of the combined network is perhaps the best organization to use if you want to understand the changes across the conditions with the oleate condition serving as the baseline.
It's important to remember that the above visual changes are being made on exactly the same network file, just with different node attribute files used to lay out the network with different node row orders. Futhermore, those differences are created simply by changing the sorting order used, specifying which edge wedges vary the fastest versus the slowest.
To wrap things up, let's look at an example of how we can use the glucose baseline version to visualize the network changes going from glucose to oleate. Consider the set of nodes that only have inputs from Adr1p (A) in glucose; the thick circle in the following figure highlights that group of nodes. In the oleate condition, most of these nodes now become targets of O, P and/or Y as well, in various combinations. The other four thin circles highlight where to look to see these changes:
 |
| Click on image to enlarge |
So, for example, about half of these glucose A-only targets become O targets as well in oleate; look at the leftmost red circle to see this. And though it is challenging with the limited resolution of these images, we can also spot two targets that go from A-only in glucose to having all four inputs in oleate. Given the node ordering, they are the two uppermost nodes in the band. You can pick them out at the very top of the P-target set (the third red highlight circle from the left).
So if you have two (or more) networks you want to compare, combine them all into one while using unique link suffix tags to tell them apart. Then use the link group feature to represent each network as separate edge wedges. Finally, change the node row ordering as needed, using the node attribute layout feature, to visualize your data from different perspectives.